Research
The research domains of the Jaseng Spine and Joint Research Institute (JSR) encompass a broad spectrum within Integrative and Korean Medicine, particularly emphasizing non-surgical interventions for spine conditions. These interventions include acupuncture, pharmacopuncture, herbal medicines, natural products, among others.
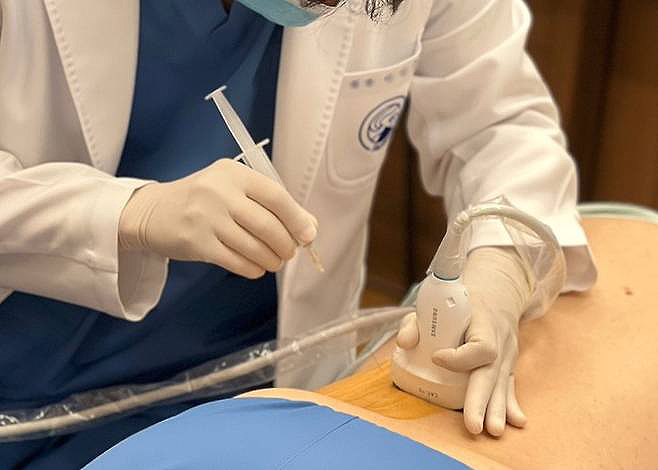
1. Clinical Research
The clinical research at JSR aims to evaluate the efficacy and safety of various treatments in integrative medicine. The key areas of focus include:
- Investigational New Drug (IND) research for the approval from the Ministry of Food and Drug Safety in the field of herbal medicine and pharmacopuncture.
- Researcher-led randomized controlled trials (RCTs), including pragmatic clinical trials (PCTs), in herbal medicine, pharmacopuncture, Chuna, and motion-style acupuncture therapy (MSAT).
- Observational studies using electronic medical records.
- Cost-effectiveness studies analyzing the economic impacts and benefits of integrative therapies.
Furthermore, JSR contributed to the following national projects in Korea to promote the standardization and development of Korean Medicine:
- Korean Medicine Clinical Practice Guidelines Development, funded by the Ministry of Health and Welfare: This project involves developing guidelines to standardize and enhance clinical practices within Korean Medicine.
- Focused Korean Medicine Research Center, funded by KoMIT: This center specializes in treatments and research centered on Korean Medicine approaches.
2. Basic Experimental Research
The JSR basic experimental research team is dedicated to conducting both in vivo and in vitro studies to uncover the mechanisms and validate the effects of various therapeutic methods in Integrative Medicine:
- Health Food Supplements and Natural Products: Investigating the potential health benefits and mechanisms of natural supplements.
- Herbal Medicine and Pharmacopuncture: Exploring both in vitro and in vivo mechanisms and effects.
- Acupuncture: Studying the physiological effects and underlying mechanisms of various acupuncture methods through in vitro and in vivo research.
In addition, the JSR basic experimental research team has developed specific animal models, including:
- Rat lumbar disk herniation model
- Rat intervertebral disc degeneration model

3. Real-World Data (RWD) Research
The RWD research in JSR is dedicated to investigating real-world data to gain insights into treatment effectiveness, safety, and utilization patterns in clinical practice:
- National Health Insurance Database: Utilizing large-scale health insurance data to analyze trends, outcomes, and policies.
- EMR Data Analysis: Leveraging Electronic Medical Records (EMR) from 21 Jaseng Hospitals to conduct comprehensive research on treatment outcomes and patterns.
- Joint database using EMRs and national health insurance database: Integrating data from both EMRs and the national health insurance database to provide a more comprehensive analysis of healthcare trends, treatment efficacy, and cost-effectiveness.

4. Jaseng Human Research Protection Center
Jaseng Spine and Joint Research Institute, along with the network of 21 Jaseng Hospitals of Korean medicine, collaborates closely with the Jaseng Human Research Protection Center (HRPC). The HRPC is responsible for the institutional review of all research conducted within the Jaseng network. Jaseng HRPC is officially certified by the Ministry of Food and Drug Safety to conduct Institutional Review Board (IRB) reviews for both human and animal studies. Additionally, it provides annual training courses for medical professionals engaged in human research.